Increase the success rate for
fertility clinics in IVF treatments
 NON-INVASIVE PGT-A
NON-INVASIVE PGT-ANon-invasive PGT-A (niPGT-A) is a genetic screening method for blastocysts grown for use in IVF treatment.
- Identifies blastocysts with an abnormal number of chromosomes.
- Helps clinicians prioritize the best blastocysts for implantation and thereby improve implantation rates and successful pregnancies.
- Is “non-invasive” as it uses the spent culture medium in which the blastocysts are grown.
CAUSES OF IMPLANTATION FAILURE
One of the primary causes of implantation failure and spontaneous abortion is an abnormal number of chromosomes (aneuploidy). Aneuploidy is common in human blastocysts and becomes increasingly common with age. On average, a woman at the age of 40 will have an aneuploidy incidence rate of more than 50%, and at the age of 44 the incidence rate is almost at 80% [1]
Unfortunately, aneuploid blastocysts are indistinguishable from euploid blastocysts in both morphology and developmental rate, making them undetectable with conventional morphological analysis [2]. As transfer of aneuploid blastocysts negatively affects the reproductive outcome, it is desirable to detect aneuploidy by other means during IVF treatment.
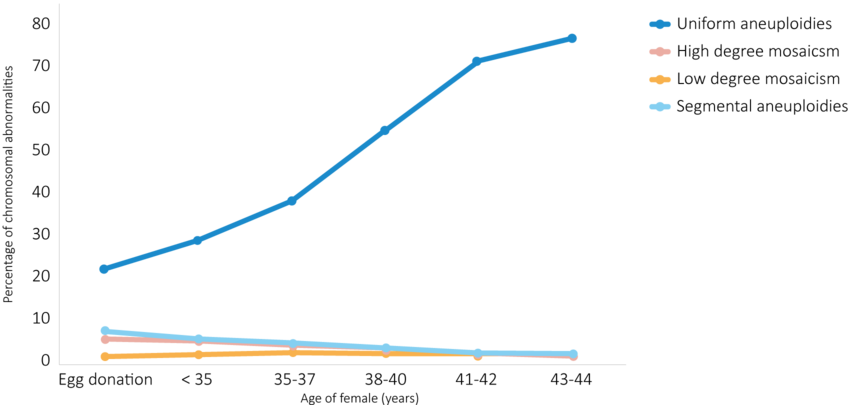
Figure 1. Frequency of chromosomal abnormalities detected in blastocysts according to female age. Uniform aneuploidies describe whole chromosome aneuploidy distributed across all biopsied cells. High degree mosaicism describes whole chromosome aneuploidy in 50-70% of biopsied cells. Low degree mosaicism describes whole chromosome aneuploidy in 30-50% of biopsied cells. Segmental aneuploidies describe aneuploidy in >10mb segments of the chromosome. (Modified from: Navarro-Sánchez et al., 2022).
THE NEW NON-INVASIVE TECHNIQUE
The most common method employed by clinics for aneuploidy detection is Preimplantation Genetic Testing for Aneuploidy (PGT-A). PGT-A can increase the number of successful pregnancies by prioritizing the blastocysts with the best chromosomal profiles. In conventional PGT-A, a biopsy of the trophectoderm is collected and embryonic DNA extracted from the trophoblasts. However, this biopsy is potentially damaging to the blastocysts and only represents the chromosome count of the trophectoderm and not the embryo itself [3].Recently, a non-invasive alternative to the trophectoderm biopsy was developed. It was discovered that blastocysts secrete cell-free DNA (cfDNA) into the culture medium in which they are grown. Using this cfDNA as a template for a non-invasive PGT-A (niPGT-A) has proven highly effective. This cfDNA has proven as representative of the actual embryo as the DNA obtained from the trophectoderm biopsy. The origin of the cfDNA is also very likely to originate from healthy blastocyst tissue, representing both the inner-cell mass and the trophectoderm [4].
niPGT-A offers a risk-free alternative to traditional PGT-A with high concordance to trophectoderm biopsies, inner cell mass and the whole blastocyst. The basis for the analysis is the spent culture medium (SCM) from the routine growth of blastocysts in IVF clinics. As SCM is collected and discarded upon vitrification of the blastocysts, incorporation of niPGT-A into the existing workflow is close to seamless.
BIOPSY FREE & GENTLE = The new niPGT-A
No damage - non-invasive.
HOW IS niPGT-A NON-INVASIVE?
The blastocyst naturally releases DNA into its culture medium. Upon vitrification of the blastocyst, the spent culture medium can be sent to Amplexa Genetics for niPGT-A analysis. At Amplexa we specialize in extracting the DNA from the culture medium and performing an aneuploidy test on this DNA. As no biopsy is required, the method is not invasive to the blastocyst.
HOW ACCURATE IS niPGT-A COMPARED TO PGT-A?
Recent studies into the concordance rate of niPGT-A compared to PGT-A have achieved great results. Part of the improvement is due to the removal of maternal contamination in the form of cumulus cells. Additionally, it has been found that the best concentration of cDNA in the SCM is found on day 5 of blastocyst growth. Taking these factors into account, niPGT-A has been found to have higher concordance rates for embryo ploidity than PGT-A (94% vs 82%) [5].
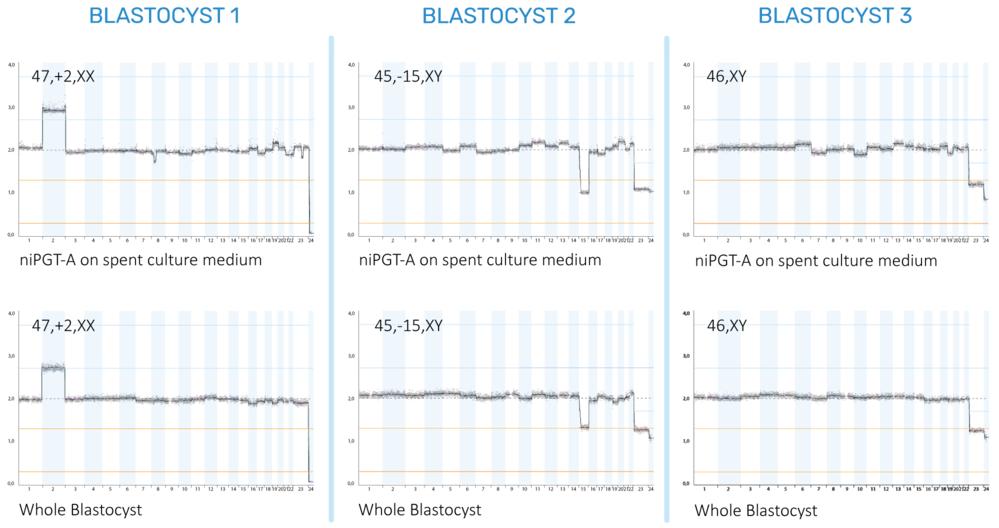
Figure 2. Chromosomal profiles based on cfDNA
from spent culture medium (SCM) and DNA extracted from the whole blastocyst.
Each column represents the same blastocyst, first tested with niPGT-A on the
SCM and then with a regular PGT-A on the entire blastocyst rather than a
trophectoderm biopsy. The whole blastocyst represents the ideal chromosomal
profile as it is based on the entire genetic profile of the inner cell mass and
trophectoderm. Our results show that the niPGT-A based on the cfDNA from the
SCM has high concordance with the whole blastocyst. Blastocyst 1 has a trisomy
on chromosome 2, Blastocyst 2 is missing one chromosome 15, and Blastocyst 3 has
a healthy chromosomal profile.
IS cfDNA REPRESENTATIVE OF THE EMBRYO?
cfDNA was previously thought to come from apoptotic aneuploid cells within the blastocysts, however, aneuploid blastocysts do not secrete more cfDNA than aneuploid blastocysts [4].
HOW ARE THE BEST niPGT-A RESULTS ACHIEVED?
To achieve the best results from an niPGT-A, we recommend the following steps during blastocyst growth: The cumulus cells must be removed, and the blastocysts washed, this is to prevent contamination with maternal DNA. If maternal DNA is present in the SCM during niPGT-A, it can be detected but also invalidates the results of the test for aneuploidy. To get sufficient cfDNA in the medium we also recommend that the blastocysts have been grown in said medium for a minimum of 24h.
WHEN IS niPGT-A RELEVANT?
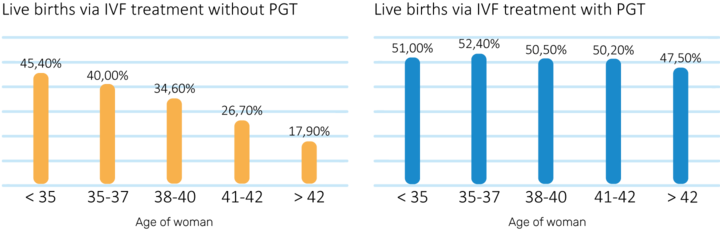
Since aneuploidy increases significantly with age, an niPGT-A analysis becomes more relevant as The patient grows older. According to data from the 2018 National Summary Report, PGT analysis is most effective in women above the age of 38 [6].
REFERENCES
[1] L. Navarro-Sánchez, C.García-Pascual, C. Rubio, and C. Simón, “Non-invasive preimplantation genetictesting for aneuploidies: an update,” Reproductive BioMedicine Online,Jan. 2022, doi: 10.1016/j.rbmo.2022.01.012.
[2] A. Capalbo et al.,“Correlation between standard blastocyst morphology, euploidy and implantation: An observational study in two centers involving 956 screened blastocysts,” HumanReproduction, vol. 29, no. 6, pp. 1173–1181, 2014, doi:10.1093/humrep/deu033.
[3] H. F. Chen, M. Chen, and H. N. Ho, “An overview of the current and emerging platforms for preimplantation genetic testing for aneuploidies (PGT-A) in in Vitro fertilization programs,” Taiwanese Journal of Obstetrics and Gynecology, vol. 59, no. 4. Elsevier Ltd, pp. 489–495, Jul. 01, 2020. doi:10.1016/j.tjog.2020.05.004.
[4] M. Vera-Rodriguez et al., “Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development,” Human Reproduction, vol. 33, no. 4, pp. 745–756, Apr. 2018, doi: 10.1093/humrep/dey028.
[5] L. Huang, B. Bogale, Y.Tang, S. Lu, X. S. Xie, and C. Racowsky, “Noninvasive preimplantation genetic testing for aneuploidy in spent medium may be more reliable than trophectoderm biopsy,” Proceedings of the National Academy of Sciences of the United States of America, vol. 116, no. 28, pp. 14105–14112, 2019, doi:10.1073/pnas.1907472116.
[6] “2018data reported by National Summary Report,” https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx
HOW TO DO IT?